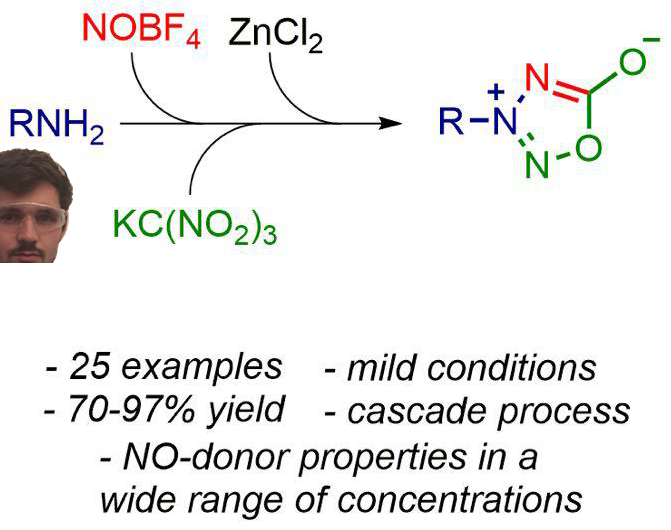
A novel one‐pot cascade method for the assembly of valuable NO‐donor azasydnone scaffold has been developed. This construction strategy involves a diazotization/azo coupling/elimination/double rearrangement cascade sequence of readily available amines. The current protocol enables the generation of a diverse array of azasydnones, including previously hardly accessible heteroaryl substituted azasydnones (25 examples, 70‐97% yield) with a good functional group tolerance under very mild conditions. Preliminary NO‐releasing studies revealed an ability of azasydnones to produce NO in a wide range of concentrations. This method provides a new approach to nitrogen‐oxygen heterocycles with potential applications in medicine and material science.
Назад к списку
Straightforward Access to the NO‐Donor Azasydnone Scaffold by Cascade Reactions of Amines